Heart failure is a serious problem in the United States that kills 1 in 9 people every year. With the rate of obesity rising and the Baby Boomer generation growing older, the number of heart failure cases is only going to increase. There is already a shortage of donor hearts in the US and some patients are stuck waiting years for a heart transplant. Can the use of design thinking help formulate a solution to this growing problem?
I took a course on the design of artificial organs during my senior year of college and was fascinated by what was being done in the biomedical engineering field. The recent development of left ventricular assist devices has given people with heart failure a new lease on life. These relatively small devices reduce the stress on the heart by helping it pump blood, and can extend a patient's life long enough to make it to a heart transplant. Though these devices perform their job well, the focus of their design has been on the engineering and not on the patient's user interaction. Because of this, there is still much room for improvement.
The Problem
Heart failure is a serious problem that affects 5.1 million people in the United States. In recent years, biomedical engineering has developed implantable devices that reduce the stress on a failing heart and extend a patient's life long enough to make it to transplant. These Left Ventricular Assist Devices (LVADs) used to be too large to implant in women and children but current devices are now small enough for most patients. However, it seems like little consideration was given to how the user actually interacts with these devices on a day-to-day basis.
The Current Solution
One of the best LVADs on the market today is the HeartMate II by Thoratec. This is a heart pump that is small enough to be implanted in men and women of smaller statures and has been clinically tested to be effective at relieving the heart. The HeartMate II includes the heart pump itself, a control monitor, and a battery system. While the heart pump works well, the whole controller and battery system offers a horrible user experience for the patient.
The Heart Pump
The HeartMate II uses an axial pump to drive blood from the left ventricle to the aorta. The impeller of the pump is magnetically levitated and rotates without any friction so it doesn't wear down over years of use. However, its rotational speed is fixed so it pumps blood at a constant rate regardless of a user's stress and exercise levels. Is it possible to design a better heart pump that can automatically adapt to a user's heart rate?
The Batteries
The whole system requires the use of two large rechargeable batteries and a mess of cords. These batteries are so large that they must be worn in a holster at all times. Additionally, the batteries last for 3 to 6 hours under normal use so most patients have to keep multiple backup batteries with them throughout the day. Each night when a patient goes to sleep, they remove the battery pack and plug into a power unit that's connected to a wall outlet. Is it possible to design a more convenient power and sleep system than this?
The Control Monitor
The control monitor is a bulky device that the user interacts with and is made from the same off-white plastic that most hospital devices are made from. This material choice makes the user feel like they have never left the hospital. What's worse is that the user must carry this large controller with them at all times and the user interface of the controller is dated. Is it possible to design a compact controller that empowers the user and follows modern design language?
The System Monitor
While the controller gives a user basic information about the battery and alarms in the event of an emergency, the system monitor gives a user detailed information about the heart pump. When connected to the controller, it shows the flow rate, RPM, and power use of the heart pump. This system adds additional cords to the already cumbersome experience and has an incredibly dated user interface. Is it possible to design a more convenient way to view detailed pump information?
A Better Heart Pump
By the end of my class on designing artificial organs I had an understanding of the engineering that went into designing a heart pump. At the same time, the design thinker in me kept suggesting that there was serious room for improvement so I decided to design a better heart pump. I was excited for the depth of this challenge because it offered a unique opportunity to apply my engineering, industrial design, and user experience design backgrounds and create a product that could truly help people.
The Premise
Studies have shown that the hearts of patients using LVADs actually begin to heal after the implant. I have a theory that continuously adjusting the heart pump to keep it at the optimal 70% value will allow patients to recover enough to no longer need a transplant. The project challenge, then, is to design a heart pump that automatically adjusts to a user's heart rate while offering a better user experience than the competition.
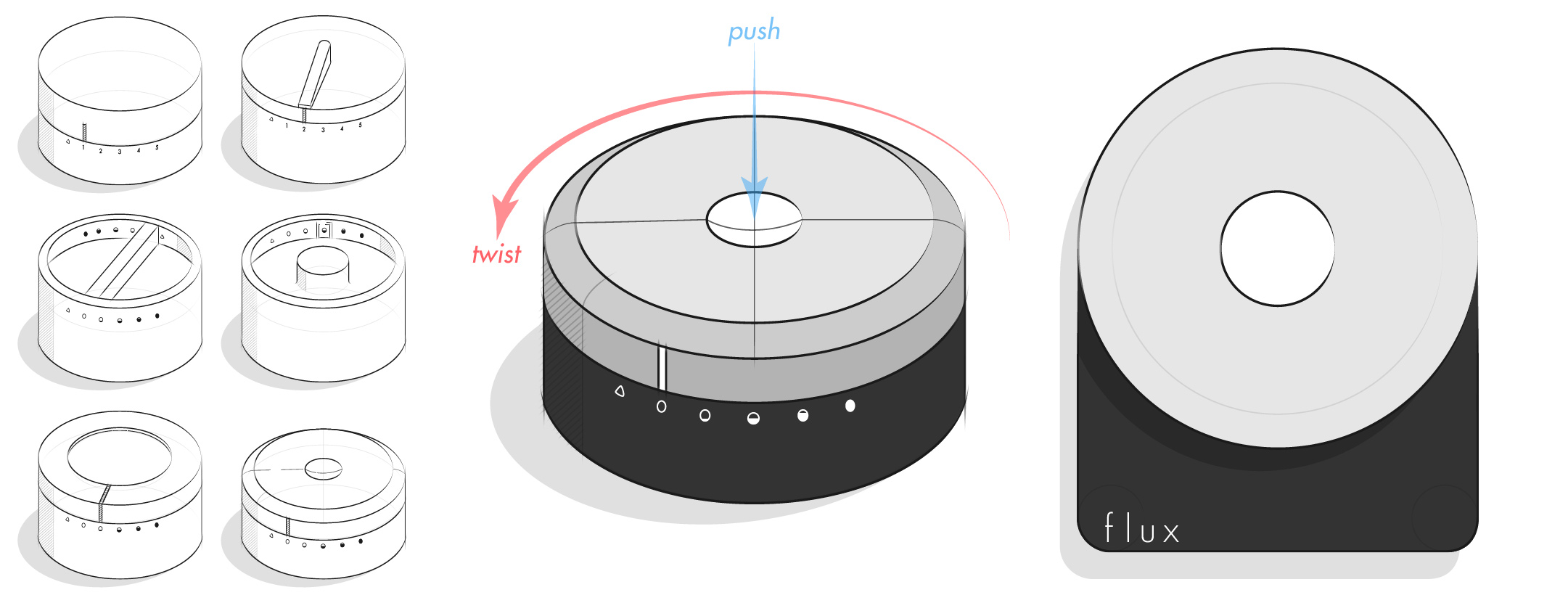
Initial Ideation
I started the ideation process by doing research on LVADs then writing ideas on post-it notes and sticking them to a wall. What emerged were various user needs and aspects of the heart pump that could be improved. The bigest improvements are outlined below.
My final solution to this design problem is called the Flux heart pump and uses four components to automatically adjust the flow rate of the LVAD. These four components are a smart watch that records a user's heart rate in real time, an app, the Flux controller, and the Flux heart pump itself. Details of this system are outlined below.
Continuous Optimal Pump Speed
The Flux LVAD system continuously adjusts the speed of the heart pump so it's at 70% of the total flow rate. This allows patients the best opportunity to heal enough to no longer need a heart transplant.
Flux App
The Flux app gives a user all of the information they need about their heart pump. It shows the current battery life, RPM, flow rate, power use of the heart pump, and offers helpful graphs that allow a user to track their recovery progress. Because most people already carry a smartphone, the Flux app does all of this without adding additional hardware. And unlike the dated user interfaces of the HeartMate II's controller and system monitor, the Flux app has a modern user interface that can be updated at any time.
Flux Controller
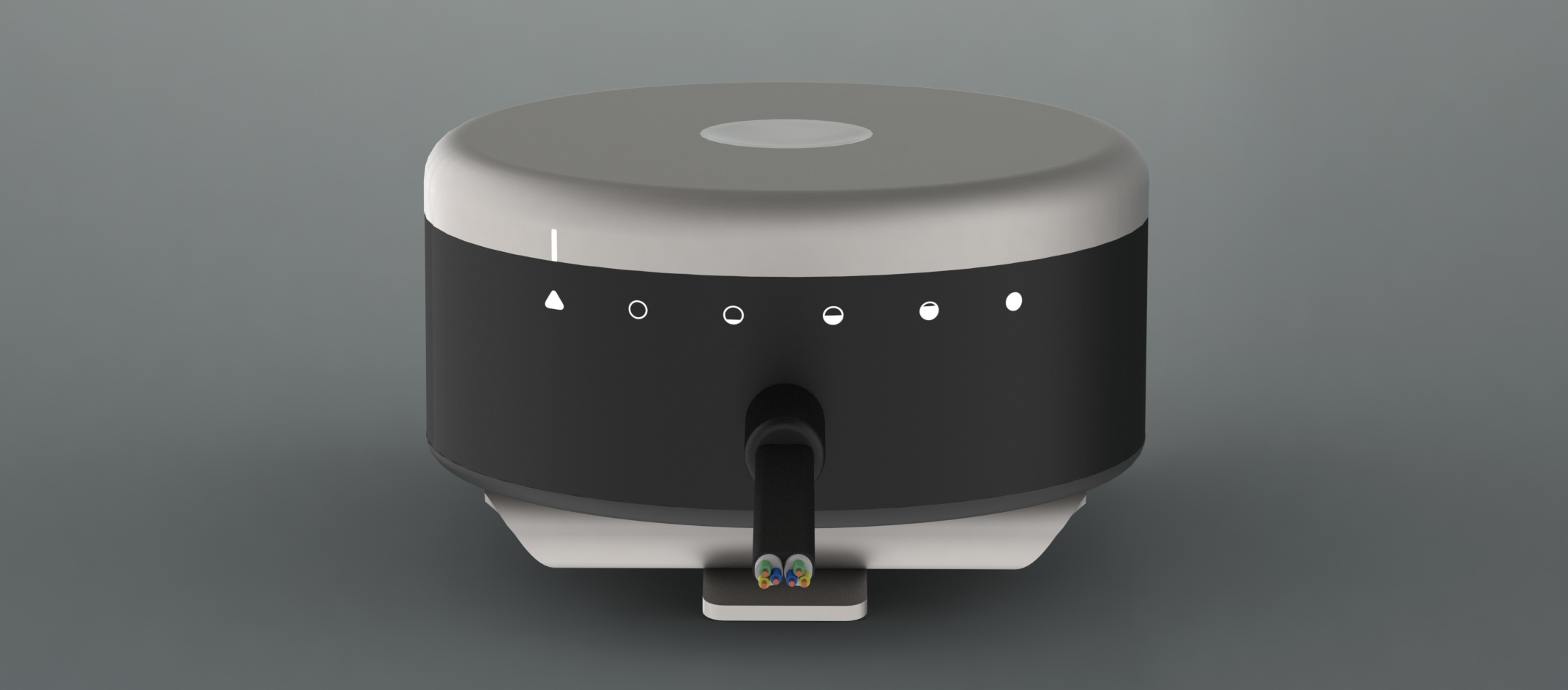
Sometimes a user would prefer to manually adjust the speed of the heart pump instead of relying on the automatic system that's in place. This is done through the flux controller. Adjusting the pump speed is as simple as pressing the white button and twisting the turntable. And unlike the HeartMate II, the Flux controller was designed to be stylish and use an internal battery instead of a battery holster.
Flux Heart Pump
The Flux heart pump is a left ventricular assist device and is what actually does the pumping so the heart doesn't have to work as hard. This device was engineered to be smaller and more efficient than the HeartMate II so it can be implanted in a wider range of people. It also has a smaller inlet and outlet tube so smaller incisions have to be made when fixing it to the heart.
Flux Sleep Sleeve
Instead of having the user plug a cord from their body into an outlet when they sleep, the Flux heart pump allows the user to sleep naturally. All they do is remove the cord from the Flux controller and plug it into the Flux sleep sleeve. This velcro battery sleeve is then attached to the user's leg or arm, depending on what's most comfortable for them to sleep in, and will keep their heart pump charged for 15 hours. When they wake up, they simply unplug from the sleep sleeve and plug into the Flux controller then begin their day.
The following sections offer an in depth look at the Flux controller, Flux app, and Flux heart pump.
The Flux Controller
The Flux controller is how a user interacts with the heart pump. Because it must be worn by a user throughout the day, special care was taken to ensure that it is treated as a stylish accessory instead of a dull piece of medical equipment. It was meant to offer the user a sense of empowerment over their condition instead of a constant reminder of their frequent hospital visits.
Mood Board
The overall mood for the Flux controller was one of refined luxury, precision engineering, and bold style. I wanted the user to feel a strong connection to their device and wear it with the pride of a stylish timepiece. Above all, I wanted the user to feel confident that, like the heart pump inside them, the controller was engineered to last.
Ideation
My goal for the controller design was to keep the interactions analog because I believed that relying on lights and buttons would make the device look cheap and quickly look dated. After experimenting with various adjustment methods, I arrived at the final design. It is composed of a black matte plastic base with a titanium adjustment knob and white plastic center button. To switch the pump setting, you simply push the center button and turn the adjustment knob to the desired level. Requiring the center button to be pushed before turning the knob ensures that a user can't accidentally adjust the pump setting.
With the design direction in place, I needed to figure out an ergonomic size for the controller. I did this by printing out 9 different sized paper prototypes and interacting with them the way a user would. From this test I concluded that a 6.5 cm dial and 5.5 cm base was best because it was small and ergonomic, yet large enough for a long battery life.
Final Product
There are three ways to wear the Flux controller: in a pocket, clipped to a waistband or belt, and clipped to a shirt. This versatility allows users more freedom to express themselves than ever before. And because special care was taken to ensure the controller is stylish, it compliments an outfit like no other LVAD.
The bulk of the Flux controller is made up of the battery. A top priority for this device was battery life, so the design maximizes the battery size and fully utilizes the internal space.
The titanium clip on the back of the controller is built to last. Because its design was inspired by a tie-clip, it is sure to keep the controller in place without damaging clothes.
There are six pump speeds that a user can choose from. When the dial is set to the triangle, the Flux controller uses Bluetooth to communicate with the smart watch and app and automatically adjusts the flow rate of the heart pump based on a user's heart rate. However, there are times when a user doesn't need or want the automatic adjustment (like wanting to conserve battery life during a flight or waiting for a phone to finish charging) and during these cases the heart pump can be adjusted to one of the 5 levels. The five filling circles represent a setting for sleeping, sitting, standing, walking, and jogging and can accommodate even the most active user throughout the day.
The Flux controller does away with the bulky battery holsters and short battery life of the HeartMate II. Instead, it uses a replaceable internal battery. The battery is both smaller and lasts longer than the HeartMate II because the Flux heart pump was engineered to be energy efficient. The heart pump itself produces the same flow rate at half the power of the HeartMate II and, because all of the calculations are performed on a smartphone and transmitted to the heart pump via low-power Bluetooth, the controller requires much less power as well. Based on my calculations, the rechargeable lithium-ion battery should last 9 hours under normal use before it needs to be switched out.
A common problem patients with LVADs encounter is accidentally snagging the cord on objects around the house. This causes the cord to yank away from the skin and often leads to an infection. The Flux controller instead uses magnetic plug connectors that pull out without yanking away from the skin. People are human so accidents are bound to happen, but infections will not.
The Flux App
The Flux app is where the behind the scenes magic takes place. It works in conjunction with a heart rate monitoring smart watch to automatically adjust the speed of the heart pump to the optimal level. And because it continuously records heart rate data, a physician can make insightful decisions regarding how various medication are interacting with a patient's heart.
Inspiration
The Flux app took its design cues from 1960's Swiss poster design. Due to their use of bold colors, modern san-serif typeface, and simple layout, these posters have remained stylish for over 50 years. On the other side of the spectrum, much of the app design seen today follows current design trends which causes them to look dated once these trends fall out of style. My hope was by using similar design principles as the posters, the Flux app would continue to look modern for years to come.
Screenshots
App Sections
Launching The App
The Flux app requires a user to log in using their email address. All of the user's heart rate data is tied to their email and saved on a secure Flux server. This allows their doctor easy access to the data and ensures a backup of the data is available in the event that the app is accidentally deleted.
Overview
The Overview section shows all the relevant pump information a user needs to know. They can see how long they have until the battery needs to be changed, the flow rate of the heart pump, and their current heart rate. Additionally, a user sees when their next pump checkup date it.
Heart Health
The Heart Health section allows a user to track their recovery. A user gains confidence that their heart is healing when the can see that their resting heart rate is steadily decreasing as treatment progresses. These graphs also allow a doctor to track how different medications interact with a patient's heart rate so they can write informed prescriptions.
Pump Statistics
The Pump Statistics section gives users detailed information about their heart pump. Giving a user access to this information allows them to track the performance of their heart pump and gain confidence that it is working correctly.
Care
The Care section puts all of the user's relevant care phone numbers in one place. Whether they need to schedule an appointment or have a sudden emergency, they can quickly make the call from this section.
Settings
The Settings section is where a user can make changes to the app. Both the stroke volume and resting heart rate get measured at each hospital visit, allowing the user to keep these values up to date within the app.
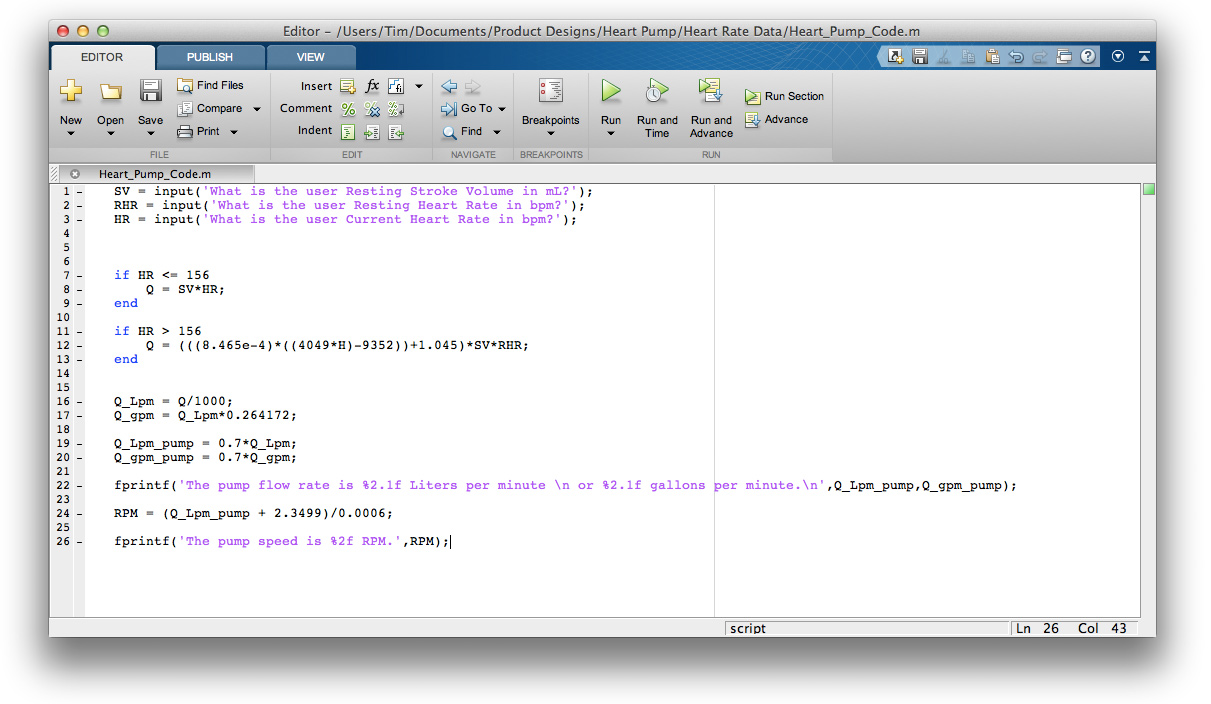
Underlying Algorithm
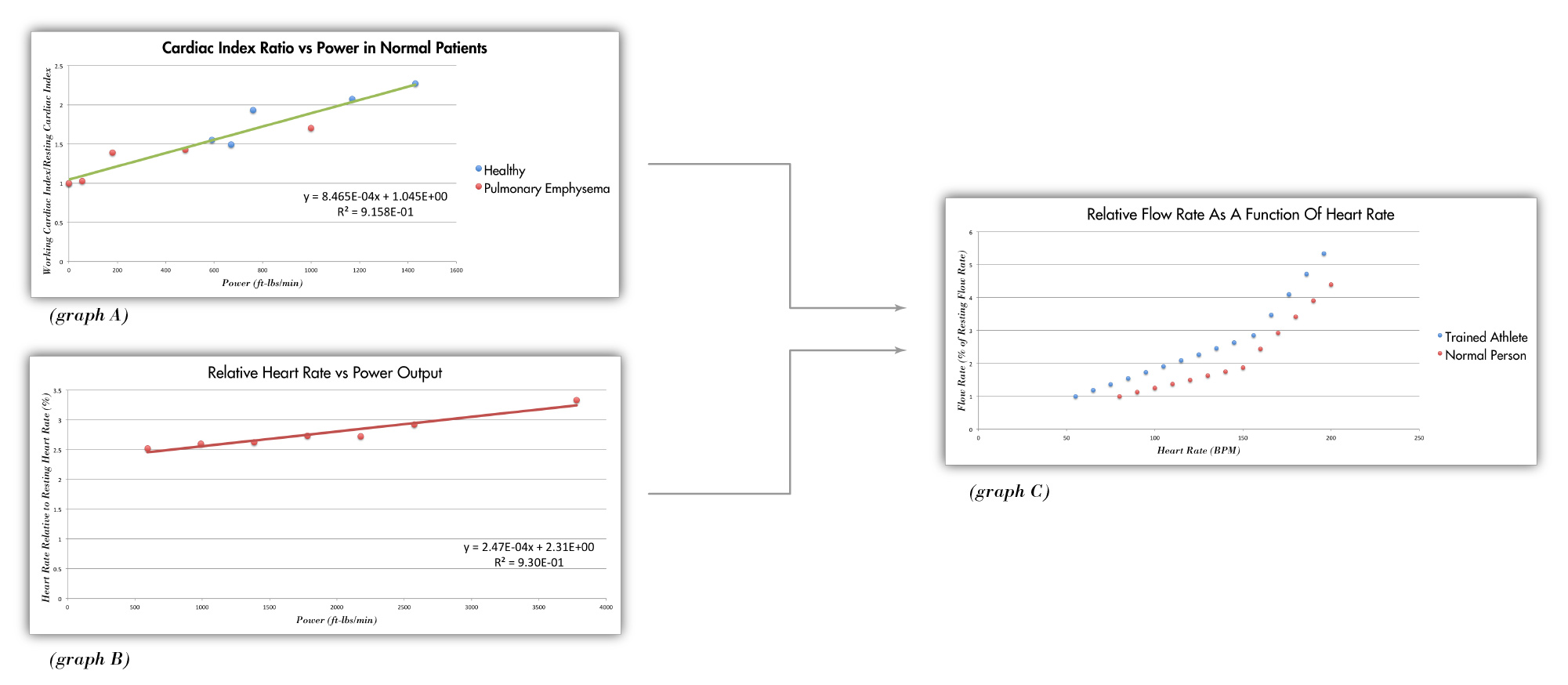
Behind the nice user interface of the Flux app is a unique algorithm that adjusts the heart pump's flow rate depending on a user's heart rate. The human body uses two mechanisms to adjust blood flow when the body's oxygen demand changes: altering the heart rate and altering the stroke volume. When a person suddenly stands up, their heart rate increases to accommodate the increased oxygen demand and when a person jogs, both their heart rate and stroke volume increase to meet the oxygen needs. Though scientists have documented that these mechanisms exist, I couldn't find a published model that empirically describes their relationship. As a result, I had to relate a few data sets to describe the model myself. This model is vital to understanding how blood flow changes as a function of heart rate and is the key to the Flux heart pump.
The first phase of the algorithm calculates a user's cardiac index based on their heart rate. I found a medical journal with data relating a person's cardiac index to their power output and, after normalizing the variables, obtained a linear relationship with an R-squared value of .916 (graph A above). I then performed an experiment where I measured my own heart rate while squatting with various weight. From this data, I was able to relate heart rate to power output and, after normalizing the variables, obtained a linear relationship with an R-squared value of .930 (graph B above). Because these are two linear equations with three variables, it's possible to relate all three variables to one another and express relative cardiac index as a function of relative heart rate. The final expression is dynamic and scales according to a user's individual resting heart rate and stroke volume. Graph C depicts this expression for two different resting heart rate and stroke volume conditions.
The second phase of the algorithm relates the pump flow rate to the pump speed. By running the computational fluid dynamics model at varying pump speeds, I was able to determine the relationship between the RPM of the heart pump and the flow rate it produces. This relationship is strongly linear with an R-squared value of .998. Finally, relating this expression to the first phase of the algorithm allows the heart pump RPM to be expressed as a function of a user's heart rate. This final relationship is expressed in the MATLAB code below.
Flux Heart Pump
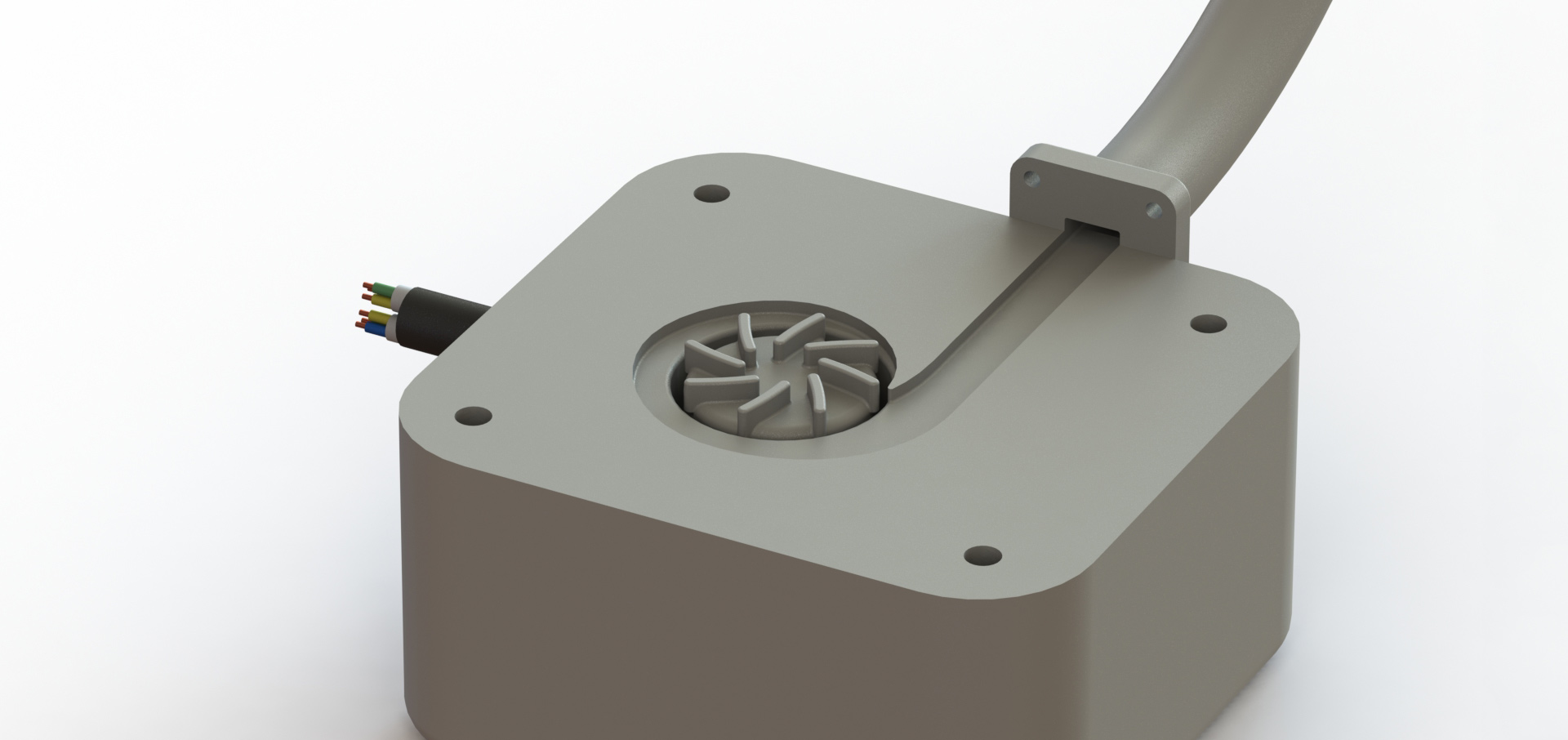
The Flux heart pump represents the next generation of left ventricular assist devices. Other than the goal of adjustable flow rate, this device was designed to be smaller and more energy efficient than the competition. Because of this, the Flux heart pump can be implanted in people with smaller body sizes and there is no need to carry around a bulky battery holster.
Final Product
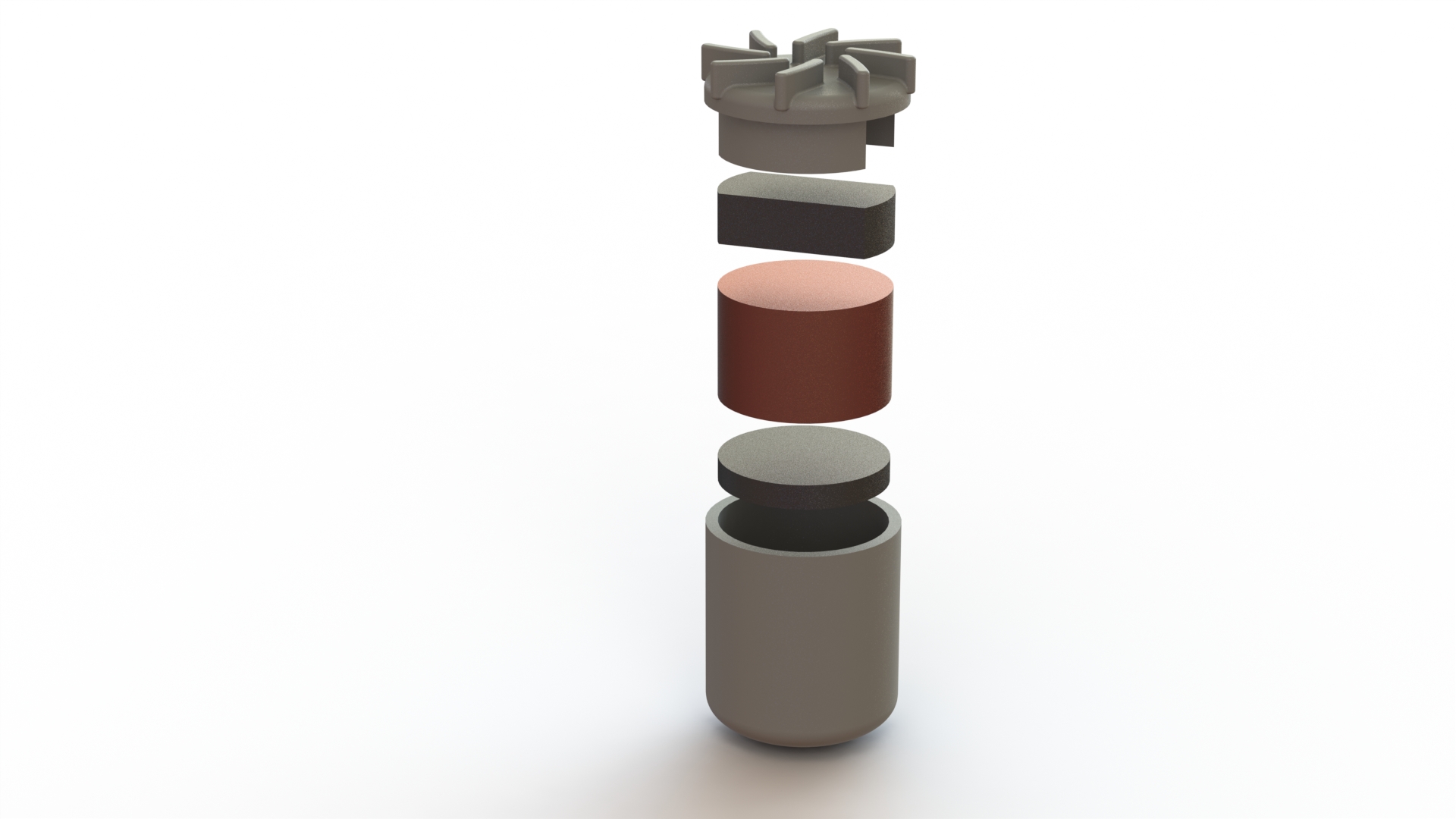
The Flux heart pump was engineered for manufacturing. It has few parts and tooling is relatively straightforward for each component. Additionally, the pump body and impeller are made from a biocompatible plastic called polyphenylsulfone. This plastic doesn't corrode in the body, is incredibly strong, can be sterilized before implanting, and can be used by 3-D printers. The 3-D printing nature of this material has the potential to drastically reduce manufacturing costs if the necessary tolerances can be met.
The driving force behind the Flux heart pump is a centrifugal impeller. When the impeller rotates, it forces blood along the outer edge of the channel which causes an increase in pressure. Because fluid flows from hight to low pressure, the blood flows from the high pressure of the impeller channel to the lower pressure of the aorta artery.
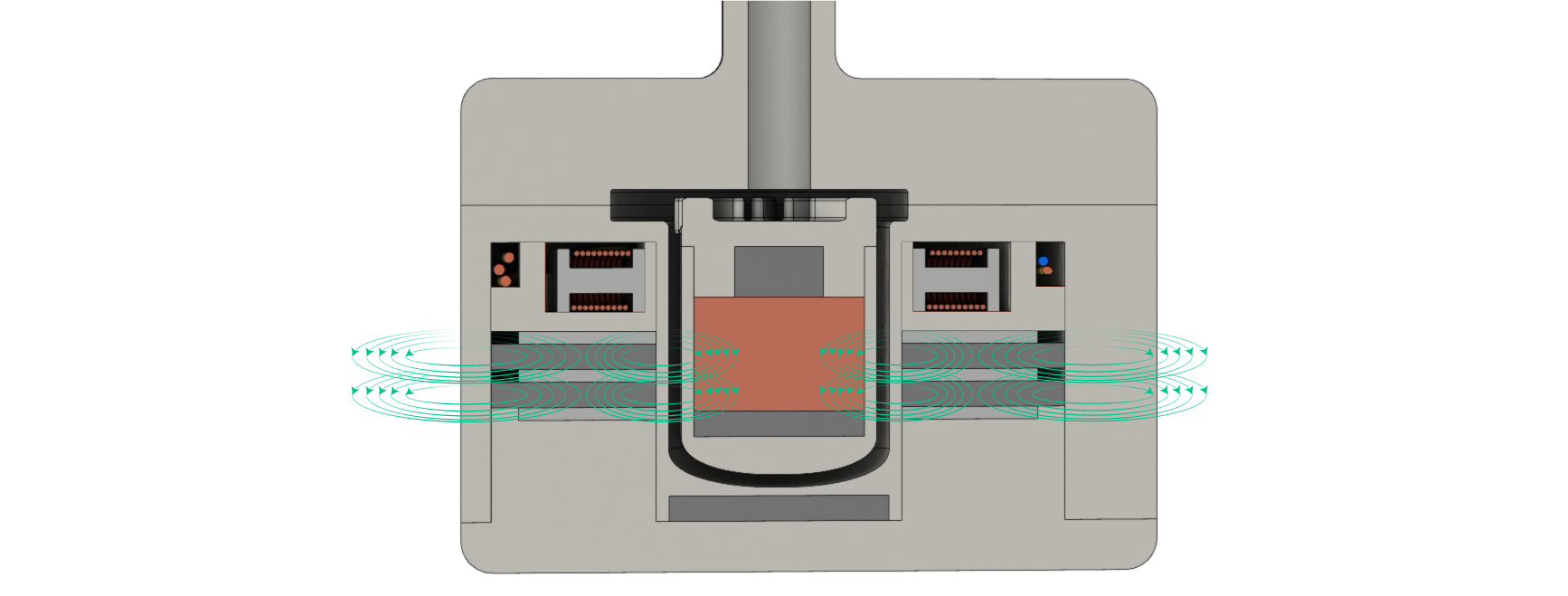
The impeller has a copper core, two internal neodymium magnets, and a polyphenylsulfone outer casing. It's the upper neodymium bar magnet that allows the impeller to be driven like a motor and it's the copper core that allows the impeller to levitate.
The impeller rotates in place due to an electromagnetic ring that effectively turns the impeller into a levitating motor. This was the hardest component to engineer because the electromagnets needed copper wire that was thin enough to allow for multiple coils yet thick enough so it wouldn't overheat and melt. After optimizing the variables, I found that using 25 gauge wire with 10 coils was able to produce a 2.25 Tesla magnetic field while remaining within the recommended safe operating temperature.
The electromagnets are wired to make adjusting the speed of the impeller as easy as possible. The whole system only needs three wires (colored blue, yellow, and green) and the electric current of each wire is identical but phase shifted. This means that adjusting the speed of the impeller is as simple as adjusting the frequency of the electric current.
The Flux heart pump is the first LVAD to use passive magnetic levitation. While the HeartMate II uses a sophisticated computer and electromagnet array to levitate its impeller, the Flux heart pump only uses stationary neodymium magnets. The physics behind the impeller's magnetic levitation works through inductance. The ring magnets surrounding the impeller produce a magnetic field that penetrates the copper core of the impeller. Because a moving magnetic field induces an electric current, there will be an induced current in the copper core while the impeller rotates. This current, in turn, induces a magnetic field in the copper core that acts to stabilize the impeller. Altogether, the impeller experiences stable levitation as long as it is rotating. And because the heart pump is always on, it never stops rotating and is always stable. The key advantage to this type of levitation compared to the complex method of the HeartMate II is the amount of power it requires. The fact that no complex stabilization calculations are being performed is they key reason why the Flux heart pump is two times more energy efficient than the the HeartMate II.
Underlying Engineering
Pump design is a very well studied field so there was a lot of data that could be used when designing the Flux heart pump. The basis of all pump design is the Cordier diagram — a diagram relating an ideal pump's specific speed to its specific diameter. Using this diagram, it's possible to optimize a pump's size if its operating speed is known. Similar to the Cordier diagram, there is also a diagram that relates a pump's specific speed to its maximum efficiency. By using both diagrams, the optimal speed and size of a pump can be determined at various efficiencies.
Naturally, a more efficient pump is better but the diagrams suggest that a centrifugal pump's speed is directly related to its efficiency. Because of this, I decided to design for a slightly lower efficiency of around .85 in order to reduce the speed of the heart pump. This meant designing the pump with a specific speed of around 0.7 and a specific diameter of around 4. Using these two values, as well as the volumetric flow rate of the pump, the outer diameter and impeller speed can be calculated.
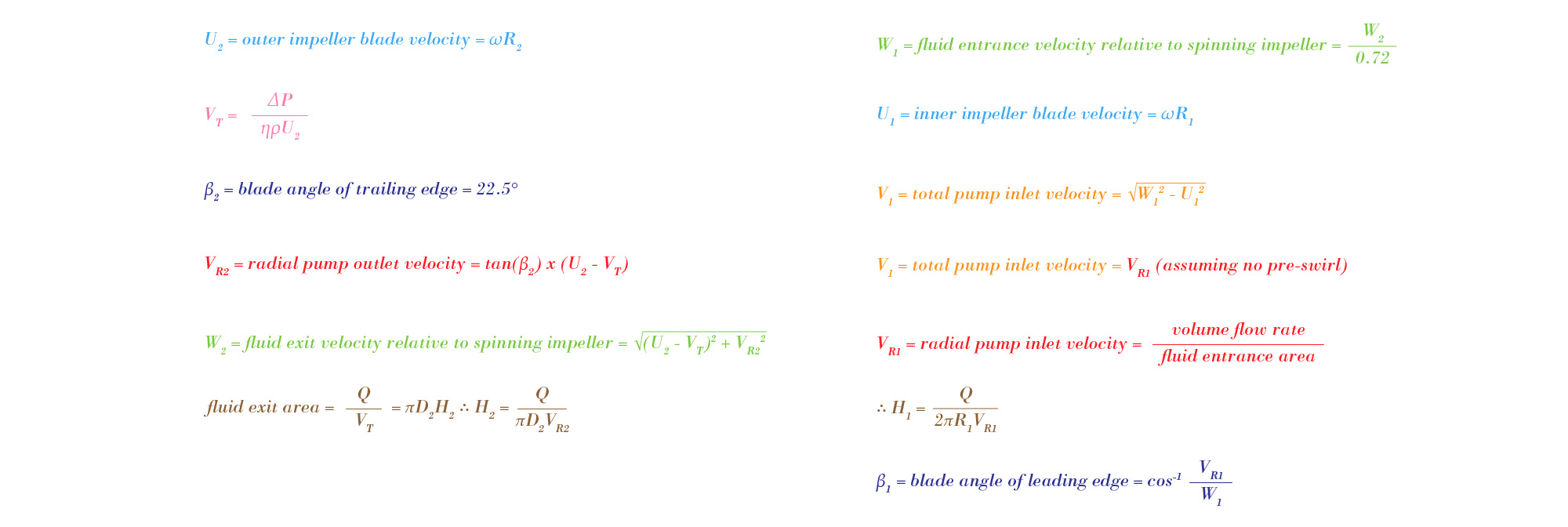
With the outer diameter and impeller speed of the pump known, the optimal dimensions of the impeller blades could be calculated. These dimensions include the inner and outer radii, the inner and outer heights, and the inner and outer blade tip-angles. In order to solve for these values, the velocity vector components of a streamline must be analyzed. The following series of equations result from this analysis.
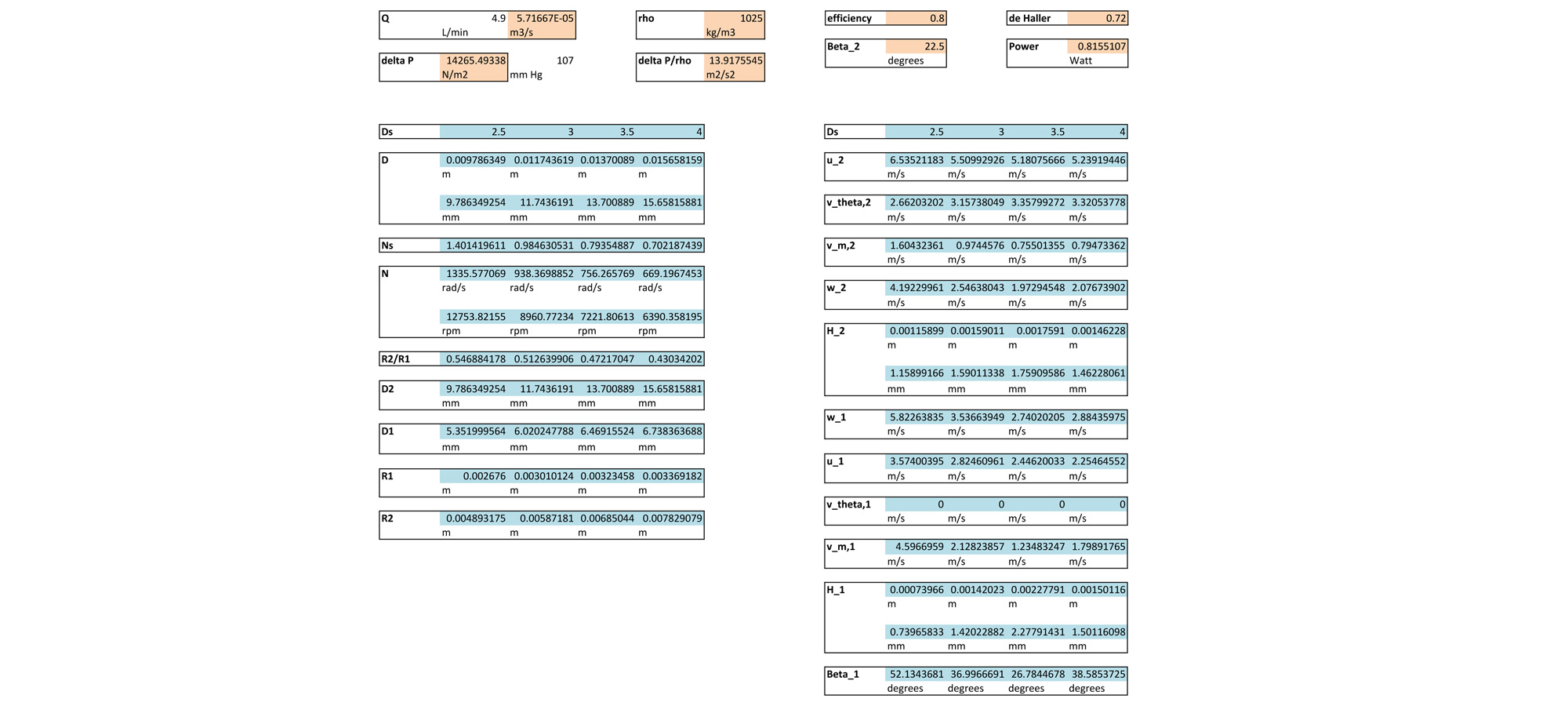
Solving this series of equations yields values for the blade radii, heights, an angles. An Excel sheet running through these calculations for various specific diameters can be seen below. The results of this spreadsheet were used to make the final SolidWorks model of the Flux heart pump.
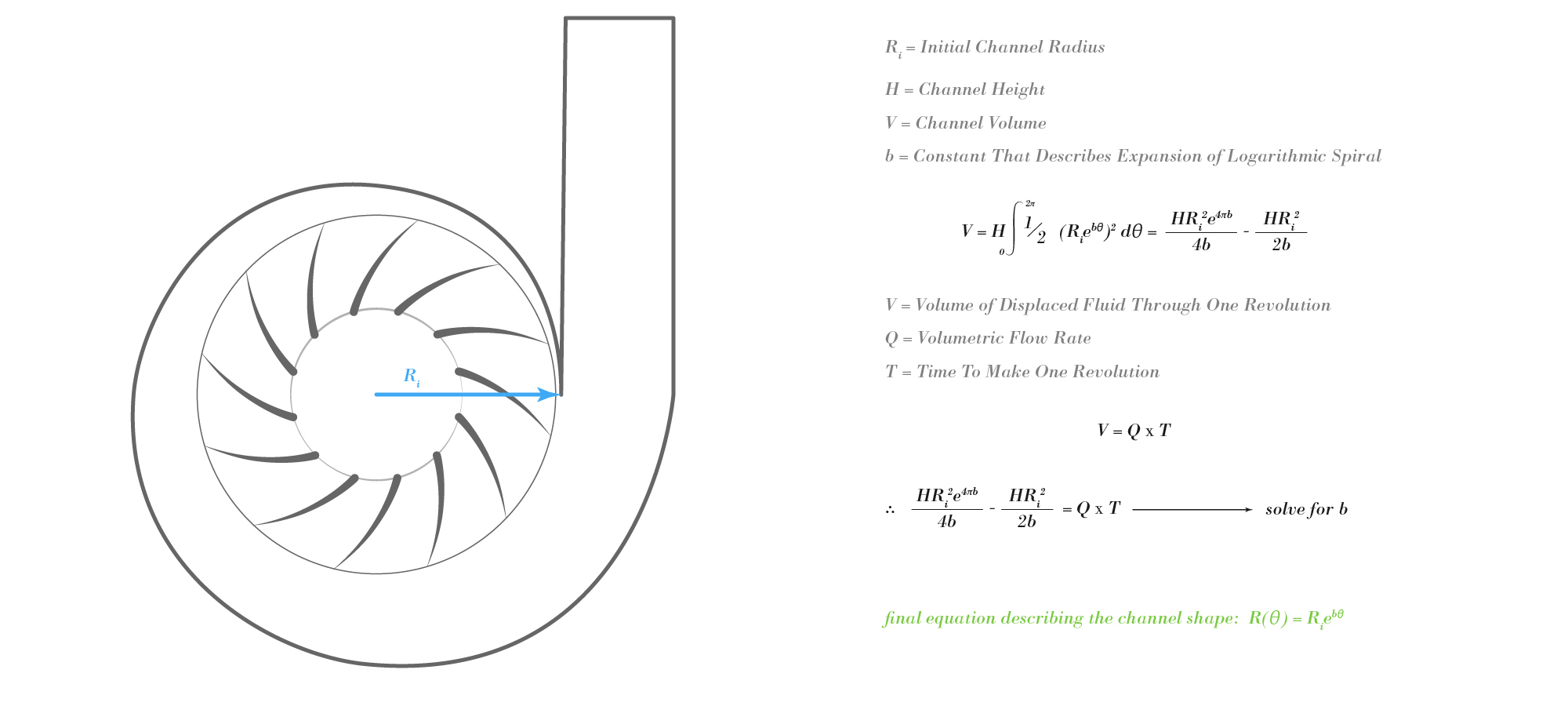
With the dimensions of the optimal impeller known, the channel that houses the impeller could be designed. Because blood is an incompressible liquid, I made the assumption that the optimal channel moves away from the impeller at the same rate that fluid is expelled from the impeller. Following this thought process, the shape of the channel should be described by a logarithmic spiral. By doing a little calculus, the constant that describes the spiral's rate of expansion can be determined. And with this constant known, the final shape of the channel can be expressed.
With all of the necessary dimensions now known, the final pump could be modeled in SolidWorks.
Computational Fluid Dynamics Results
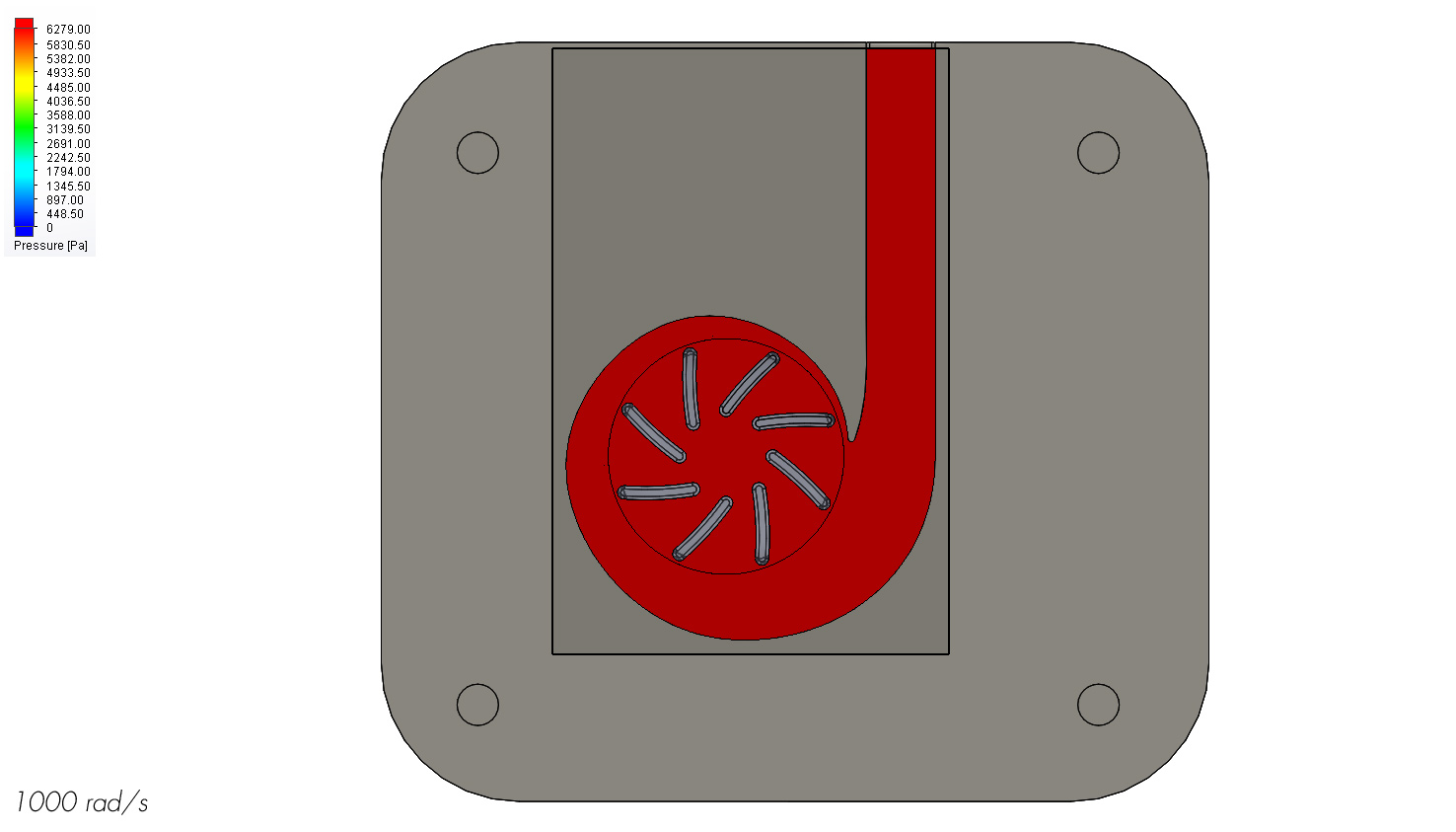
I ran computational fluid dynamics analyses in SolidWorks at various pump speeds to gain an understanding of how the heart pump would perform. It turns out that an operating speed of 1000 rad/s produces a flow rate of around 4.2 L/min. When taking into account the extra 30% flow being pumped by the heart, this equates to a total of 6 L/min. The average flow rate of a healthy resting heart is around 5-6 L/min so this puts the pump in that target.
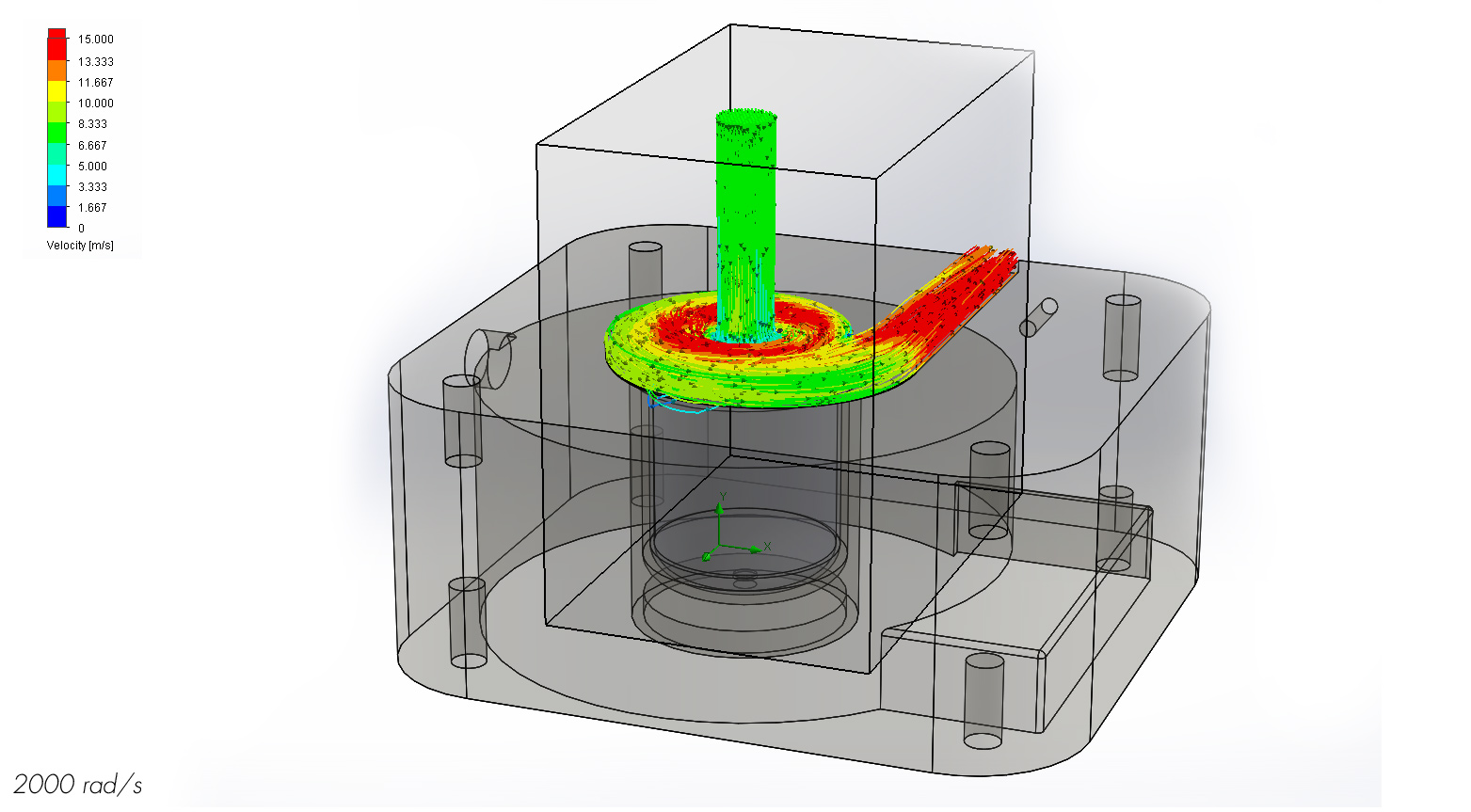
When analyzing the streamlines of the fluid at 1000 rad/s, it is clear that the impeller is doing its job. The fluid, for the most part, enters the inlet tube, rapidly rotates around the impeller, and exits the outlet tube. However there is some flow that circulates around the impeller before leaving the outlet which could potentially remain stagnant long enough to produce a blood clot. Because the computational fluid dynamics analysis is only meant as a guideline for fluid behavior, a model would have to be produced to see how long blood would actually circulate around the impeller and if it is long enough to form a clot.
At 1000 rad/s the impeller does a nice job of accelerating the fluid without producing noticeable stagnation spots along the main channel. This means the channel shape is well designed.
The streamlines at 2000 rad/s suggest a much cleaner flow than at 1000 rad/s. There are no recirculation spots so a blood clot is most likely not a problem at this speed, but a physical model should still be tested to confirm these results.
Shear stress is a major factor that needs to be considered for a blood pump. Platelets can activate and red blood cells can rupture if exposed to the conditions colored pink in the graphs above. The computational fluid dynamics determined the maximum and average shear stress that the blood would experience going around the impeller but it's difficult to tell conclusively if platelet activation and red blood cell rupture will be a problem or not because we don't know how long the max shear stress lasts for. However it's reasonable to assume that because the average shear stress is relatively low, we should not expect much platelet activation or red blood cell rupture. That being said, a physical model should be tested to confirm this assumption.
A potential problem for a blood pump is damage through cavitation. Cavitation occurs when the fluid pressure drops below the vapor pressure and causes tiny bubbles to form and subsequently collapse to produce a shockwave. Given the temperature of blood, cavitation should occur if the fluid reaches a pressure below around 6280 Pa. As the pressure map shows, none of the fluid is below this pressure so cavitation should not be an issue. However, a physical model should still be produced to confirm these results.
Moving Forward
Though the current version of the Flux heart pump is a good starting point, there is always room for improvement. The first course of action moving forward would be producing a prototype of the heart pump to test how closely the real world results follow the computational fluid dynamics model. Once these results are in hand, further refinement of the channel and impeller would be done to reduce shear stress and mitigate the incident of clotting. Finally, once the shear stress and clotting are minimized, additional refinement would be made to reduce the operating speed of the impeller and make the pump as small as possible.